Learning Outcomes
By the end of this lesson, students should be able to:
i. Explain the concept of combustion and identify the products formed during the complete and incomplete combustion of hydrocarbon-based fuels.
ii. Recognize the primary pollutants released from the combustion of hydrocarbon-based fuels, including carbon oxides (COx), sulfur oxides (SOx), nitrogen oxides (NOx), and volatile organic compounds (VOCs).
iii. Describe the environmental and health impacts of each type of pollutant, highlighting their contributions to air pollution, acid rain, smog, and greenhouse gas emissions.
iv. Identify the sources of these pollutants, including power plants, vehicles, and industrial processes.
v. Appreciate the importance of emission control technologies and clean energy alternatives in reducing pollution from combustion sources.
Introduction
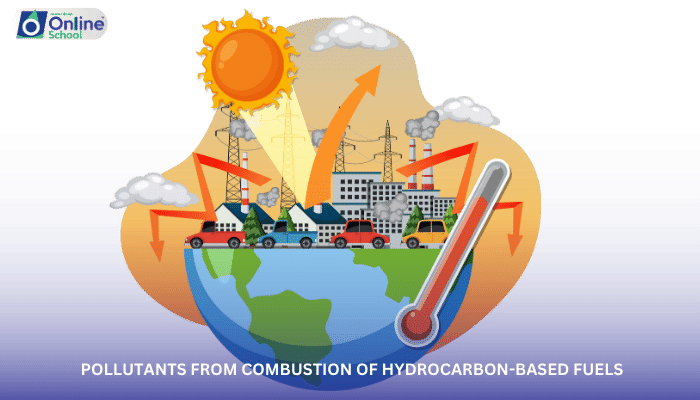
The combustion of hydrocarbon-based fuels, such as gasoline, diesel, and natural gas, is a fundamental process that powers our world, providing energy for transportation, electricity generation, and industrial processes. However, this reliance on combustion comes at an environmental cost, as the burning of these fuels releases a range of harmful pollutants into the atmosphere. This lesson delves into the invisible threat posed by pollutants from combustion, exploring their sources, impacts, and potential mitigation strategies.
i. Carbon Oxides (COx): A Silent Threat
Carbon oxides (COx), primarily consisting of carbon monoxide (CO) and carbon dioxide (CO2), are major pollutants released from combustion processes.
Carbon Monoxide (CO): A colorless, odorless gas that is highly toxic to humans. CO displaces oxygen in the blood, leading to hypoxia, a condition characterized by oxygen deprivation.
Carbon Dioxide (CO2): A greenhouse gas that contributes to global warming. CO2 traps heat in the atmosphere, leading to a rise in global temperatures and associated climate change impacts.
ii. Sulfur Oxides (SOx): A Source of Acid Rain
Sulfur oxides (SOx), primarily sulfur dioxide (SO2), are formed from the combustion of sulfur-containing fuels, such as coal and heavy oil.
Sulfur Dioxide (SO2): A colorless gas with a pungent odor that can irritate the respiratory system. SO2 contributes to acid rain, a form of precipitation with a pH lower than normal, which can damage ecosystems and human-made structures.
iii. Nitrogen Oxides (NOx): Smog and Greenhouse Gas Emissions
Nitrogen oxides (NOx), primarily nitrogen dioxide (NO2), are formed from the combustion of air and fuel in high-temperature environments.
Nitrogen Dioxide (NO2): A reddish-brown gas with a pungent odor that can irritate the respiratory system. NO2 is a major component of smog, a mixture of pollutants that can cause respiratory problems and reduce visibility.
Greenhouse Gas Emissions: NOx can also contribute to greenhouse gas emissions through indirect reactions that lead to the formation of tropospheric ozone, a greenhouse gas.
iv. Volatile Organic Compounds (VOCs): A Diverse Group of Pollutants
Volatile organic compounds (VOCs) are a diverse group of organic chemicals that easily evaporate at room temperature. They are emitted from a wide range of sources, including combustion processes, industrial processes, and the use of solvents and paints.
Environmental and Health Impacts: VOCs can contribute to photochemical smog formation, ozone depletion, and the formation of hazardous air pollutants. Some VOCs, such as benzene, are known carcinogens.
v. Sources of Combustion-Generated Pollutants: A Pervasive Threat
Combustion-generated pollutants are released from a variety of sources, including:
Power Plants: Power plants that burn fossil fuels, such as coal and natural gas, are major sources of CO2, SO2, NOx, and VOCs.
Vehicles: Gasoline- and diesel-powered vehicles are significant emitters of CO, CO2, NOx, and VOCs.
Industrial Processes: Industrial processes, such as manufacturing, metal production, and chemical processing, release various pollutants, including SO2, NOx, and VOCs.
vi. Mitigation Strategies: A Path Towards Cleaner Skies
Reducing the emission of combustion-generated pollutants requires a multifaceted approach:
Emission Control Technologies: Implementing emission control technologies, such as catalytic converters and scrubbers, can significantly reduce pollutant emissions from power plants and vehicles.
Clean Energy Alternatives: Transitioning to cleaner energy sources, such as renewable energy sources like solar and wind power, can reduce reliance on fossil fuels and their associated emissions.
Emission Standards: Enforcing stricter emission standards for power plants, vehicles, and industrial processes can drive technological advancements and reduce the overall level of pollution.
The pollutants released from the combustion of hydrocarbon-based fuels pose significant environmental and health threats. Understanding the sources, impacts, and mitigation strategies of these pollutants is crucial for addressing.